Our Mission
The mission of our laboratory is to create biologically inspired in vitro platforms, to capture the scale of cell signaling in tissue microenvironments from subcellular to tissue levels, and discover novel therapeutics for human diseases.
We are particularly interested in creating microfabricated models of cancer and stem cell microenvironments, and developing integrated techniques for biosensing of microenvironmental cues. Our goal is to identify new targets and critical pathways in driving disease and intervention, and translate our discoveries into applications for immune and cancer therapeutics, cancer biomarker/drug development, and regenerative medicine.
We are particularly interested in creating microfabricated models of cancer and stem cell microenvironments, and developing integrated techniques for biosensing of microenvironmental cues. Our goal is to identify new targets and critical pathways in driving disease and intervention, and translate our discoveries into applications for immune and cancer therapeutics, cancer biomarker/drug development, and regenerative medicine.
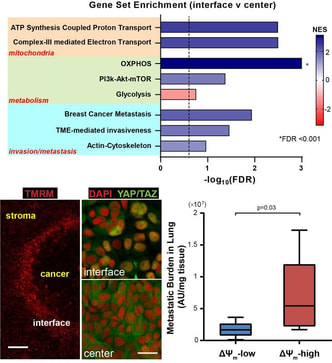
Mitochondrial Heterogeneity and Metastasis
We are developing micropatterned tumor models to study the impact of mechanical cues in tumor microenvironment on the mitochondrial heterogeneity of cancer cells and the implication in cancer metastasis. (Image: RNA sequencing shows higher mitochondrial activities at the tumor stromal interface (top); mechanosensors YAP/TAZ are co-regulated with mitochondrial membrane potential (bottom left); mitochondrial membrane potential correlates with metastatic potential in a cancer cell line in vivo (bottom right)) (Begum et al., Sci Rep, 2019) Immunotherapy in Hypoxic Tumors
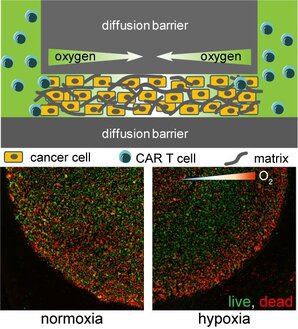
Using micro-technologies, we are creating tumor-on-a-chip devices to understand how chimeric antigen receptor (CAR) T cells infiltrate and attack cancer cells in a hypoxic tumor microenvironment, and how to augment the therapeutic efficacy. (Image: illustration of hypoxic tumor model (top) and CAR-T cell induced cancer cell deaths in a normoxic (left) or hypoxic tumor microenvironment (right)) (Ando et al, Adv Healthc Mater, 2019) |
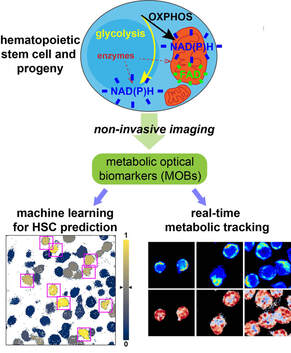
Metabolic Optical Biomarkers for Hematopoietic Stem Cell and Leukemia
Using fluorescence lifetime imaging microscopy (FLIM) and machine learning tools, we are developing non-invasive metabolic optical biomarkers (MOBs) and feature libraries to track stem cell differentiation and leukemia drug responses at the single-cell level. (Image: Cell metabolism is reflected by the redox states of FAD and NADH, captured by FLIM (top); HSCs can be identified by MOBs through a machine learning model (bottom left) and tracked of functional changes during in vitro culture (bottom right)) (Zhou et al., iScience, 2020) Hematopoietic Stem Cell-Niche Interactions
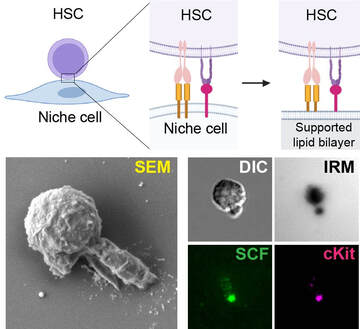
We are developing artificial bone marrow niches to understand how HSCs interact with niche stromal cells, to improve their in vitro expansion and clinical transplantation. (Image: supported lipid bilayer mimicking membrane-bound interactions between HSCs and niche stromal cells; unique polarized HSC morphology induced by membrane-bound factors) (Hao et al., J Cell Biol, 2021) |
Opportunities
Graduate and undergraduate students interested in joining the lab should email a CV and short description of research interests to Prof. Shen. Interested undergrads should have a minimum GPA of 3.5 (or equivalent, for freshmen) and be able to commit >=10 hours/week between 9am ~ 6pm.
1042 Downey way, los angeles, ca 90089 department of biomedical engineering Viterbi school of engineering
© 2017 Laboratory for integrative biosystems engineering. All Rights Reserved.